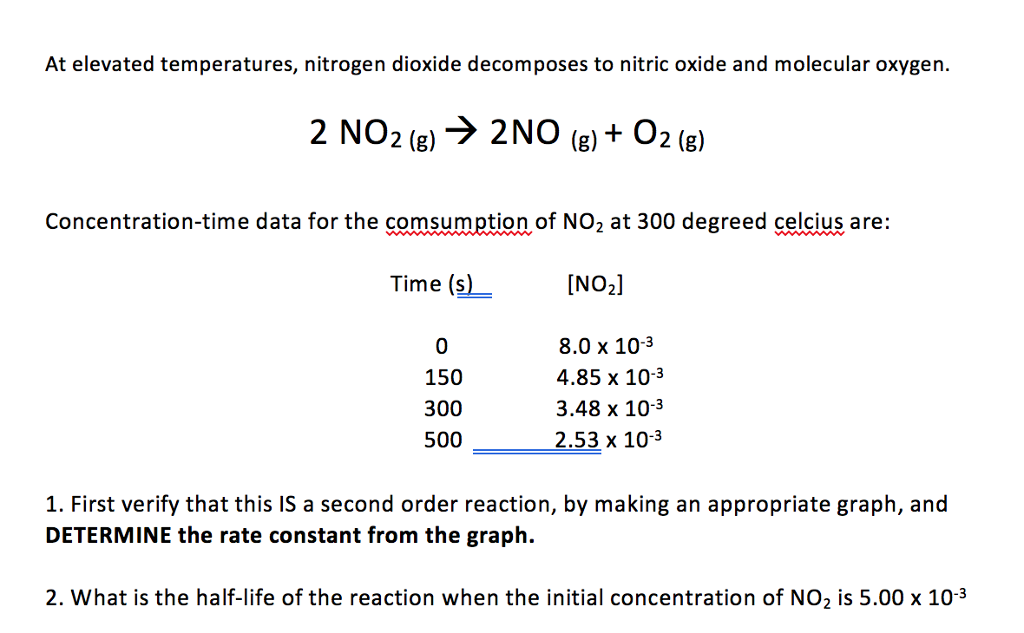
Nitrogen Dioxide Decomposes At 300 . at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. The decomposition reaction was monitored in. It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ). nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide and oxygen according to the. nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2no2 → 2no + o2 in a particular experiment at 300 °c,. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. nitrogen dioxide (no 2) is a reactive oxide of nitrogen.
from www.chegg.com
2no2 → 2no + o2 in a particular experiment at 300 °c,. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ). nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide and oxygen according to the. nitrogen dioxide (no 2) is a reactive oxide of nitrogen. at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. The decomposition reaction was monitored in.
Solved At elevated temperatures, nitrogen dioxide
Nitrogen Dioxide Decomposes At 300 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. 2no2 → 2no + o2 in a particular experiment at 300 °c,. The decomposition reaction was monitored in. It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ). nitrogen dioxide (no 2) is a reactive oxide of nitrogen. at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide and oxygen according to the. nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction:
From www.numerade.com
SOLVED Nitrogen dioxide to nitric oxide and oxygen via the reaction 2NO2 2NO 02 In Nitrogen Dioxide Decomposes At 300 The decomposition reaction was monitored in. nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide and oxygen according to the. 2no2 → 2no + o2 in a particular experiment at 300 °c,. It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ).. Nitrogen Dioxide Decomposes At 300.
From www.bartleby.com
Answered 1. Nitrogen dioxide to… bartleby Nitrogen Dioxide Decomposes At 300 nitrogen dioxide (no 2) is a reactive oxide of nitrogen. at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. 2no2 → 2no + o2 in a particular experiment at 300 °c,. nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2no 2 (g) → 2no (g) + o 2 (g) if a flask. Nitrogen Dioxide Decomposes At 300.
From www.chegg.com
Solved 39. Nitrogen dioxide according to the Nitrogen Dioxide Decomposes At 300 at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide and oxygen according to the. nitrogen dioxide (no 2) is a reactive oxide of nitrogen. The decomposition reaction was monitored in. 2no2 → 2no + o2 in a particular experiment at 300 °c,. It decomposes at. Nitrogen Dioxide Decomposes At 300.
From www.numerade.com
SOLVED Nitrogen dioxide, NO gi Is produced as by product of the combustion of fossil fuels in Nitrogen Dioxide Decomposes At 300 nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: The decomposition reaction was monitored in. 2no2 → 2no + o2 in a particular experiment at 300 °c,. nitrogen dioxide (no 2) is a reactive oxide of nitrogen. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. nitrogen dioxide, $\mathrm{no}_{2},$ decomposes. Nitrogen Dioxide Decomposes At 300.
From www.chegg.com
Solved 4. Nitrogen dioxide to nitric acid and Nitrogen Dioxide Decomposes At 300 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. The decomposition reaction was monitored in. nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: nitrogen dioxide (no 2) is a reactive oxide of nitrogen. 2no2 → 2no + o2 in a particular experiment at 300 °c,. It decomposes at 300 °c. Nitrogen Dioxide Decomposes At 300.
From www.reddit.com
[College Chemical Nitrogen dioxide to nitric oxide and oxygen. According Nitrogen Dioxide Decomposes At 300 nitrogen dioxide (no 2) is a reactive oxide of nitrogen. The decomposition reaction was monitored in. nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide and oxygen according to the. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. 2no2. Nitrogen Dioxide Decomposes At 300.
From www.youtube.com
Second Order of Nitrogen Dioxide YouTube Nitrogen Dioxide Decomposes At 300 nitrogen dioxide (no 2) is a reactive oxide of nitrogen. nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide and oxygen according to the. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: The decomposition reaction was monitored in. It. Nitrogen Dioxide Decomposes At 300.
From www.chegg.com
Solved At high temperatures nitrogen dioxide into Nitrogen Dioxide Decomposes At 300 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ). 2no2 → 2no + o2 in a particular experiment at 300 °c,. nitrogen dioxide decomposes to nitric oxide and. Nitrogen Dioxide Decomposes At 300.
From www.numerade.com
SOLVED At elevated temperatures, nitrogen dioxide to nitrogen oxide and oxygen NOz Nitrogen Dioxide Decomposes At 300 The decomposition reaction was monitored in. nitrogen dioxide (no 2) is a reactive oxide of nitrogen. nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. It decomposes at 300 °c. Nitrogen Dioxide Decomposes At 300.
From www.chegg.com
Solved 3. Nitrogen dioxide at 300°C via a Nitrogen Dioxide Decomposes At 300 at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. The decomposition reaction was monitored in. nitrogen dioxide (no 2) is a reactive oxide of nitrogen. It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ). 2no2 → 2no + o2 in a particular experiment at 300 °c,. nitrogen dioxide decomposes to. Nitrogen Dioxide Decomposes At 300.
From holooly.com
At elevated temperatures, nitrogen dioxide to nitric oxide and molecular oxygen 2 Nitrogen Dioxide Decomposes At 300 at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2no2 → 2no + o2 in a particular experiment at 300 °c,. nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide and oxygen according to the. The decomposition reaction was monitored in. nitrogen. Nitrogen Dioxide Decomposes At 300.
From www.chegg.com
Solved Nitrogen dioxide at high temperatures Nitrogen Dioxide Decomposes At 300 nitrogen dioxide (no 2) is a reactive oxide of nitrogen. at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. 2no2 → 2no + o2 in a particular experiment at 300 °c,. It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ). nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide. Nitrogen Dioxide Decomposes At 300.
From www.numerade.com
SOLVED At elevated temperatures nitrogen dioxide to nitrogen oxide and oxygen. The Nitrogen Dioxide Decomposes At 300 at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. nitrogen dioxide (no 2) is a reactive oxide of nitrogen. It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ). nitrogen dioxide decomposes to nitric oxide and oxygen. Nitrogen Dioxide Decomposes At 300.
From www.bartleby.com
Answered 7. Gaseous nitrogen dioxide bartleby Nitrogen Dioxide Decomposes At 300 nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. nitrogen dioxide (no 2) is a reactive oxide of nitrogen. The decomposition reaction was monitored in. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. nitrogen dioxide, $\mathrm{no}_{2},$ decomposes. Nitrogen Dioxide Decomposes At 300.
From www.chegg.com
Solved At 330°C nitrogen dioxide according to the Nitrogen Dioxide Decomposes At 300 2no2 → 2no + o2 in a particular experiment at 300 °c,. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ). nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide and oxygen according to the. at high temperatures,. Nitrogen Dioxide Decomposes At 300.
From www.chegg.com
Solved 1. The following data were obtained for the gas phase Nitrogen Dioxide Decomposes At 300 nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. The decomposition reaction was monitored in. 2no2 → 2no + o2 in a particular experiment at 300 °c,. nitrogen dioxide (no. Nitrogen Dioxide Decomposes At 300.
From www.youtube.com
of of nitrogen pentoxide Chemical Physical Chemistry YouTube Nitrogen Dioxide Decomposes At 300 It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ). nitrogen dioxide, $\mathrm{no}_{2},$ decomposes upon heating to form nitric oxide and oxygen according to the. 2no2 → 2no + o2 in a particular experiment at 300 °c,. The decomposition reaction was monitored in. nitrogen dioxide (no 2) is a reactive oxide of nitrogen. . Nitrogen Dioxide Decomposes At 300.
From www.numerade.com
SOLVED Nitrogen dioxide to nitric oxide and oxygen via the reaction 2NO2 (g) → 2NO Nitrogen Dioxide Decomposes At 300 It decomposes at 300 °c into nitric oxide (no) and oxygen (o 2 ). nitrogen dioxide (no 2) is a reactive oxide of nitrogen. at high temperatures, nitrogen dioxide decomposes to nitric oxide and oxygen. 2no 2 (g) → 2no (g) + o 2 (g) if a flask that. 2no2 → 2no + o2 in a particular experiment. Nitrogen Dioxide Decomposes At 300.